What is the INA-CRR?
The Indonesia Clinical Research Registry (INA-CRR) is an online registry of clinical research being undertaken in Indonesia. The INA-CRR includes studies from the full spectrum of therapeutic areas of pharmaceuticals, surgical procedures, preventive measures, lifestyle, devices, treatment and rehabilitation strategies, and complementary therapies.
The INA-CRR on the process to being recognized by the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) as a Primary Registry. WHO recognizes registries as Primary Registries if they fulfil certain criteria with respect to data content, quality and validity, accessibility, unique identification, technical capacity, and administration.
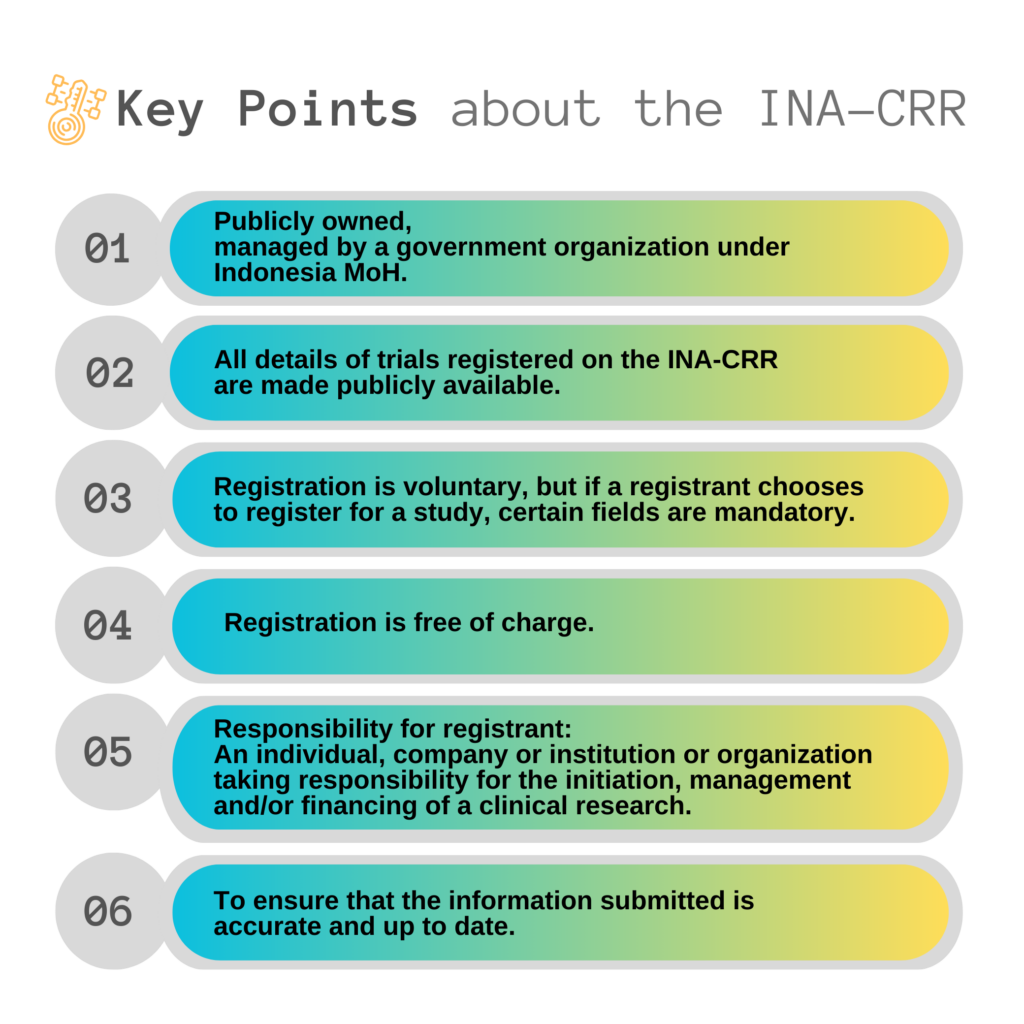

The INA-CRR mandatory data items comply with the minimum dataset requirements of the International Committee of Medical Journal Editors (ICMJE) and the World Health Organization (WHO). Once the submitted data complies with these requirements, it will be allocated a unique registration number.
